The Copycat Invasion

India’s cancer patients at risk from unauthorised, unapproved, illegally imported generic copy “medicines”
The incidence of cancer is growing exponentially. The main factors are an actual increase in incidence due to our lifestyle choices, increased longevity and the willingness of patients to come forward for diagnosis and treatment. The availability of government schemes like Ayushman Bharat and other state-level programmes also make it possible for the underprivileged to get free treatment for cancer and other important diseases. As new developments continue to bring forth better treatment options, their cost can remain a significant deterrent (e.g. robotic surgery, Cyberknife radiation therapy, and Car-T-Cell medical therapy). This is because advances can only happen after companies incur significant costs in proving that new medicines or technology is effective and safe.
The patient-centric focus has always been on improving the chances of cure, optimizing survival and maintaining quality of life among cancer patients. Newer medicines have made this possible in ways that we could not imagine a decade ago. Today, oral medicines are increasingly available for targeting DNA mutations that are “driving” cancer to grow and spread. Also, the advances in the field of immuno-oncology are making available drugs that are capable of rejuvenating the patients’ immune system. Our previous issue included interviews with the two stalwarts who received the Nobel Prize in Medicine last year for their contributions towards advancing our understanding of immune-oncology.
I started thinking about this problem after a chance discussion with a colleague from Chennai. He did not know how to respond to the request from his patient to approve Olanib, which was manufactured in Bangladesh and there was no evidence of it being approved by DCGI for marketing in India. I decided to conduct a survey of fellow oncologists. I was shocked by the results. Patients had access to unproven “medicines” and there was a marketing blitz from across our borders. It seems that either no one knew about the extent of the problem or that no one was aware of this economic and healthcare attack on our country.
Dr Purvish Parikh Group Oncology Advisor, Shalby Cancer & Research Institute, India
Parikh PM et al conducted an online survey among top medical oncologists of India (personal communication, manuscript submitted to a medical journal). This was focused on their personal experience of exposure to information and current status about ‘generic copies’ of oral high-end oncology drugs (like osimertinib and afatinib) in India. They were asked to give simple yes/no answers to three questions:
1. Have you experienced your patient discussing, asking questions regarding or purchasing generic/copy oral medicines like osimertinib or afatinib?
2. Was such a copy drug supplied to them through their government-supported or funded scheme (eg ECHS, CGHS, ESIS)?
3. Have you been contacted by a ‘marketing’ person willing to provide such generic copy imports to patients in India?
They received a total of 99 unique answers.
| Q. No. | Yes | No |
| 1 | 62 | 37 |
| 2 | 02 | 97 |
| 3 | 41 | 58 |
Those who said yes to question three were contacted personally for further details. Of these 41 senior medical oncologists, 22 replied with details, 12 did not reply and the remaining 7 said they did not remember who had contacted them. The 22 replies with details included a total of 16 unique names and phone numbers of people providing such medicines. In addition, there were instances of unknown persons contacting the doctors via landline, WhatsApp and Facebook. Most (19/22) of the oncologists contacted were practicing in metro cities and the remaining three were from tier two cities.
Other novel marketing strategies used by them include contacting potential patients directly via posts on social media, one-to-one conversations through medical shops outside cancer hospitals and even providing a “second income” opportunity to medical representatives of pharmaceutical companies. It is estimated that, in India, about 90 to 100 patients a month are receiving a generic copy of osimertinib/afatinib alone. Even at a conservative estimate, this means that in 2018, 1,080 patients were exposed to fake generic medicines that put their lives at risk. It is said that between India and China, this grey market is as big as INR 500 crore per month, says Natrajan Ganesh, a senior pharma expert from India.
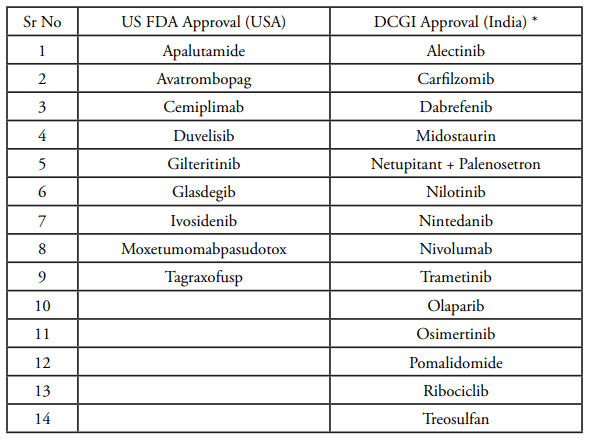
In the last two years, new key regulatory approvals by drug authorities in USA and India in the field of oncology/hematology included (in alphabetical order):
Even oncologists in tier two cities have not been spared from this onslaught. I feel the GS1 standard used in healthcare is the best way of verifying whether any particular drug is genuine or fake. This standardised system has been in use for a long time and is applicable across the world. Checking on the DCGI and CDSCO website is also recommended. We should do everything in our power to ensure that fake generic medicines are not allowed to enter our country. We should protect our patients from such dangers.
Bharath Rangrajan, Senior Medical Oncologist, Koval Medical Centre, Coimbatore

Oncology & Haematology drugs that received regulatory drug authority approval in 2017 and 2018
Unconfirmed information is that many such oral ‘high-end’ medicines for cancer are manufactured in secret at unknown locations. They are then labelled as manufactured in Sri Lanka and exported. For instance, the Lucius Pharmaceuticals website (www. luciuspharmaceuticals.com) gives its address as No. 68/186, Talakotuwa Garden, Polhengoda Junction Colombo – 05. We looked it up on Google Maps and found instead, Ilma International Girls School at that location. When we investigated further by asking a Sri Lankan colleague to physically visit the address, no such company could be found. When we contacted the phone number (+94112358489) listed on its website, it was answered by a bank. Emails to the official email address (luciuspharmaceuticals@asia. com) remain unanswered. Clearly, this is a deep mystery. Apparently, some international pharmaceutical giants who hold the patent for the original molecules have also filed complaints with concerned drug regulatory authorities. Another name floating around is that of Theodore Pharma Pvt Ltd Co, whose address is Mumbai. Its website shows specialty products including dasatanib and sorafenib.
However, it claims to only be an R&D company, is 100% export-oriented and does not sell any medicines under their name within India. Hence, allegations of its involvement are unsubstantiated. The Bangladesh story is quite different. While Square and Beximco Pharmaceuticals seem to be genuine companies not involved in fake medicines, this cannot be said for some others. Of interest are Everest, Beacon, Incepta, SP Labs and Indeed. These claim to be providing high-end oncology medicines at a fraction of the cost that the innovator company is selling for. A simple Internet search for generic crizotinib shows several hits, including the popping up of Beacon’s name.
I cannot understand how someone can be so evil as to sell more than 30 varieties of fake medicines online using a fake address and phone number. Using raw material meant for laboratories and passing it off as real medicine is a serious crime. Who will protect our patients?
Padmaj Kulkarni Senior Medical Oncologist, Deenanath Mangeskar Hospital, Pune

The Everest story is shocking and an extremely blatant example of capitalization on the grey market. It is targeting doctors and patients from across the border using WhatsApp, email, social media, etc. Patients have even sent their oncology doctors’ images of the cartons to verify if the medicines provided in India are acceptable. The packaging seems to be of high quality. The printing quality is also similar to that of the innovator companies. The image of authenticity is enhanced by using barcodes, batch numbers, manufacture date, expiry date, and even holograms. A fancy hologram today costs a few rupees and there are lakhs of suppliers easily available. Today, barcodes can be generated and read by mobile phone apps. When we tried to inspect the barcodes, it only showed a serial number. There were no internationally accepted norms of country code, product code, batch number or other details.
Barcoding for healthcare follows GS1 standards, which are open, global, proven, simple and in use for more than 30 years. Open, technology-independent standards allow full compatibility across the world. Pharma companies are not obliged to use costly and limited proprietary solutions. This is important because healthcare has universal global applications. This international standardized system allows traceability from the manufacturer of the medicine right up to the patient taking the medicine. In addition, a global trade item number (GTIN) is required to clearly identify that product in any country with ease. The fake generic medicines sold from Bangladesh do not comply with this requirement.
When I was first contacted by Mr. Anwar of Everest Pharma, Bangladesh, I thought it was a hoax. But then I was shocked to hear that he had also contacted several other colleagues by phone and WhatsApp. When a patient asks me about the cheap medicines from Bangladesh, I categorically tell them not to risk their lives by using drugs that have not been licensed by any authority or tested on human beings. I tell them exactly what I would do if I was in their place.
Dr G S Bhattacharyya Senior Medical Oncologist from Kolkata

Dr Parikh called his oncology colleague in Bangladesh to verify. The answer was that these medicines are not available in Bangladesh at all. They have also not been approved by their drug authorities. Further investigations show that there are no publications, no human patient studies, not even pharmacokinetic (PK) and pharmacodynamic(PD) studies in healthy subjects. Some of the oncologists said that their patients surreptitiously taking generic osimertinib actually seemed to benefit. How could this happen? On careful investigation, we found that some ‘manufacturers’ are using active ingredients supplied by companies like Sigma.
Such intermediaries are only for laboratory use and are not cleared for human consumption. Unethical companies might be using this raw material to manufacture fake generic medicines that are not meant for humans, let alone having been tested as required by drug authorities across the world. We have no idea what active ingredient, preservative, stabilizer, filler or impurities these fake generic ‘medicines’ contain. All that we know is that thousands of Indians are risking their lives by being exposed to such unlicensed, untested and unverified ‘drugs’. It may even be that deaths have occurred either due to toxicity or due to the underlying disease growing in the absence of real medicines.
Is this an economic war being waged by a few unscrupulous companies from across our borders? Who will protect our patients?
Also read about