Screening Out The Cancer

UE Lifesciences makes early breast cancer detection easy, painless and affordable with its handheld device, iBreastExam
By Joann Jose
Breast cancer, just like any other form of cancer, largely remains nonexistent for people until it affects someone near and dear. This was the case for Mihir Shah and Matthew Campisi, the founders of UE LifeSciences (UELS). Both of them had close family members affected by breast cancer and this prompted them to take a closer look at the magnitude of the problem and study it from a wider angle. At a time when incidences of breast cancer diagnosis are rising and survival rates are poor, UELS focuses on innovating and articulating science into commercial healthcare solutions for the developing world. UE Lifesciences was established with a mission to effectively tackle breast cancer by making early detection a reality for women. Apart from making it accessible to women across the developing world, Shah and Campisi wanted to ensure that the test was affordable to all as well. Upon further evaluation, they strongly felt that the primary reason for the high death rates associated with breast cancer was the lack of effective early-stage detection.
Like the saying, ‘Not everything turns out perfect the first time around,’ initially, the duo was not successful in devising an accurate modus operandi to tackle breast cancer. “Our first innovation was a large and expensive device. Despite its clinical accuracy, the device did not answer the requirements of the developing world. So we had to start from scratch to develop a product that was more appropriate in delivering the impact that we were seeking. This finally led to our brainchild, iBreastExam,” says Campisi. Since its launch, the device has enabled more than 60,000 women across India to take the iBreastExam test, and around 500 early-stage lesions were detected. Shah reminisces how UELS was born: “In 2009, Matt and I have bootstrapped entrepreneurs with a mission to change how women accessed breast cancer screening in the developing world. With moral and monetary support coming only from my dad and an angel investor, the early years were hard. While working on our first innovation we saw a promising technology being developed at Drexel University. Without any source of funding, we took an ambitious call and licensed that technology in 2010. Two years later, the game changed when we won the prestigious CURE grant from the Pennsylvania Department of Health and we shifted our focus to developing iBreastExam into a commercial product. That marked the first step in the commercial development of iBreastExam.” The US Food and Drug Administration clearance for iBreastExam in 2015 caught the attention of Dr Ranjan Pai (Aarin Capital), and impact investor, Unitus Seed Fund. Finding the technology and utility of the product immensely promising, they came on board as UELS’ first investors in a $3 million Series A round. A year later, UELS raised $1.2 million from Dr. Kiran Mazumdar-Shaw and Unitus in a strategic round, winning the support of one of the most prolific businesswomen the nation has seen.
“Once women are made to understand the benefits of iBE, they are keen to know where they can get themselves screened at an effective cost”.
Matthew Campisi
UELS has developed, clinically validated and commercialized two FDA cleared and CE marked devices—NoTouch BreastScan and iBreastExam, for breast cancer screening and diagnosis. Their flagship innovation, iBreastExam (iBE) is the world’s first US FDA cleared and CE marked mobile health solution for early detection of breast lumps. The handheld, battery-powered and wireless SCREENING OUT THE CANCER device is used by a number of public and private health agencies. iBE offers affordable, painless and radiation-free breast health examination using patented tactile sensor technology. “The device, operated by community health workers in settings like hospital OPDs, physician’s clinics, homes, and rural primary health centers, delivers real-time results at the point of care,” explains Shah. Compared to mammography, which is unsustainable owing to a paucity of radiologists and limited clinical utility in young women, iBE is much more practical as a screening tool. Additionally, it does not require an experienced radiologist for test administration and interpretation.
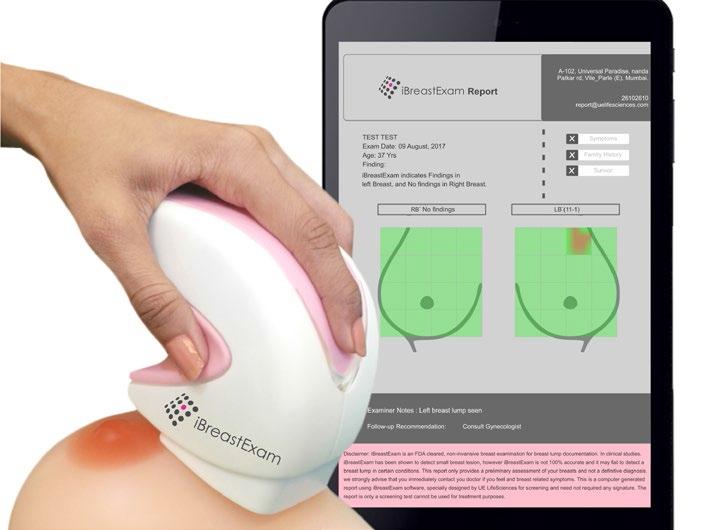
The tactile sensor technology that enables the working of iBreastExam was developed at Drexel. The sensor measures the contrast in elastic modulus between normal and abnormal breast tissues. The technology works around the fact that breast lumps or lesions are biologically stiffer and denser than healthy breast tissues. Once the sensor is calibrated to a woman’s healthy breast tissue, the device immediately identifies an area in the woman’s breasts with suspicious stiffer mass. Shah and Campisi received a massive response for their innovation. Among the 60,000-plus women screened, a generous number has unanimously praised the quick, painless and radiation-free experience. However, the duo feels that awareness among the general public is minimal. “Once women are made to understand the benefits of iBE, they are keen to know where they can get themselves screened at an effective cost,” says Campisi.